Antibiotic Stewardship: FDA Cites Progress
While the issue of antibiotic use in food animals won’t disappear anytime soon, the industry can claim significant progress toward addressing public concerns.
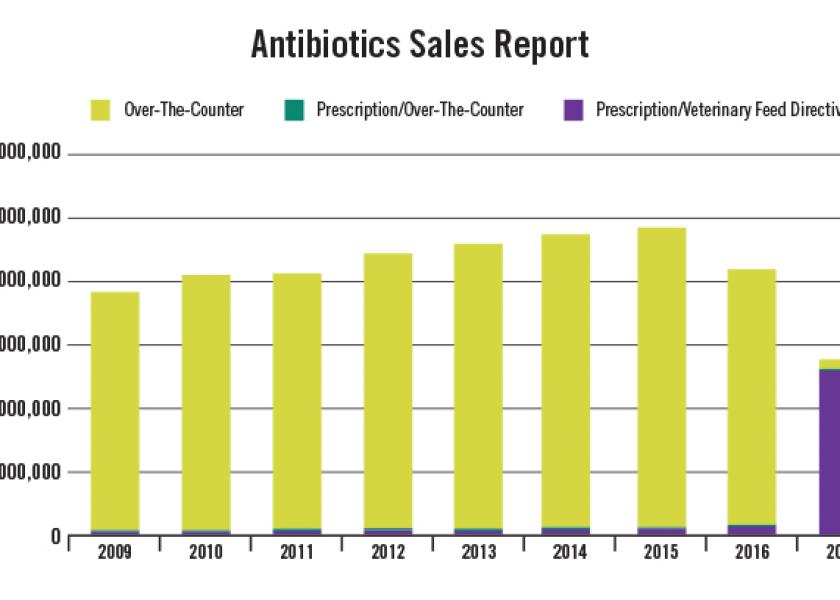
U.S. sales of medically important antimicrobials for use in food animals dropped considerably during 2017 according to the latest summary report from the FDA. The decline accelerated a trend that began in 2016 and is likely to continue, although probably not at the same annual pace as in 2017.
According to the 2017 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals, sales and distribution of all medically important antimicrobials decreased 33 percent decline between 2016 and 2017, 41 percent since 2015 (peak year of sales/distribution) and decreased 28 percent since the first year of reported sales in 2009.
The downward trend comes as no accident, as 2017 was the first full year for implementation of the FDA’s Guidance for Industry 213, which eliminated performance or production claims from medically important antimicrobials used in food animals, and the new VFD rules, which placed access to medically important antimicrobials used in feed under the oversight of veterinarians.
The report also shows a slight decline in sales of non-medically important antimicrobials for food-animal use during 2017. Cattle account for the largest portion of non-medically important antimicrobial use – primarily ionophores used for production and/or therapeutic purposes. Sales of non-medically important antimicrobials for use in cattle dropped 1% during 2017, while those for use in hogs declined 7% and chickens by 13%.
Of the 2017 domestic sales and distribution of medically important antimicrobials for use in food-producing animals, tetracyclines accounted for the largest share at 64%, followed by penicillins at 12%, macrolides at 8%, sulfas at 5%, aminoglycosides at 5%, lincosamides at 3%, and cephalosporins and fluoroquinolones each for less than 1%. The authors estimate that 42% of the total was intended for use in cattle, 36% for use in swine, 12% for turkeys, 5% intended for chickens and an estimated 5% intended for use in other or unknown species.
Veterinarians see positive trends, ongoing challenges
Veterinarian Dee Griffin, at West Texas A&M University, notes that much of the decline in use since VFD came into effect most came from the “medically important” antibiotic (MIAB) category, but non-medically important antibiotic (NMIAB) use is down 10%. More than 80% of the NMIAB are ionophores, Griffin says, adding that the FDA, CDC and WHO are adamant ionophores have no relationship or influence on humans.
Two thirds of sales of MIAB for food animals are tetracyclines, Griffin says, and less than 10% of all MIAB used fit into the FDA’s definition as “critically important to humans.” He also points out that more than 60% of MIAB reported were used in feed and about two-thirds of those were tetracyclines, with chlortetracycline (CTC) representing the bulk of the tetracycline use in feed. Feed tetracyclines only have disease “control” or “treatment” approvals, therefore not only was there no use for “production purposes,” and 2017 sales of tetracyclines would have been for humane care of animals.
Griffin also points out that water use accounts for about 30% of MIAB sales, and water medication is rarely used in cattle. Only about 6% of MIAB sales are injectable products, which he says drives home the FDA’s concern for no extra-label use of feed antibiotics for fear of massive impact of mistakes.
Kansas State University veterinarian Dan Thomson says the report further demonstrates that veterinarians, nutritionist, farmers and ranchers are working hard to judiciously use antibiotics while protecting animal health and welfare. “The beef industry has an unbelievable track record and story to tell about decreasing antibiotic residues and resistance which all started from the Beef Quality Assurance program in the 1980s. I am not surprised that we once again see another step forward by our industry.”
Thomson would like to see more detail in the FDA reporting, such as designations for beef or dairy use, rather than a combined “cattle” category, and information on herd size. “Antibiotics used, diseases and production practices are different in dairy operations compared to beef operations. If we only monitor sales of antibiotics, an increase in cattle on feed or in the milking string could increase antibiotic sales without any increase in antibiotic usage per head or per product produced.” Simply monitoring sales, he adds, could be misleading due to end-of-year specials to purchase products or other promotional activities including rebates to distributors and veterinarians.
Thomson also notes the report documents a large increase in macrolide usage. This was, he says, due to the double labeling of tylosin that is fed to control liver abscesses in feedlot cattle. The label of tylosin says to include it in the ration at eight to 10 grams per ton (g/t) feed it to provide 60 to 90 milligrams per head per day (mg/hd/d). But when tylosin was first approved, cattle had lower consumption levels. The VFD regulation informed us to feed tylosin at the label dose of eight to 10 g/t. Prior to the VFD, producers were feeding tylosin at six to eight g/ton to deliver 60 to 90 mg/hd/d. Increasing to that label dose resulted in modern cattle with large intakes receiving more 90 mg/hd/d. Since this report,
Thomson says, FDA has recommended cattle feeders focus on the 60 to 90 mg/hd/d when feeding tylosin. “In the end,” he says, “the label needs modified but that is the reason we saw the marked increase in tylosin in fed cattle.”
Jessica Laurin, DVM, with Animal Health Center of Marion County in Kansas, agrees that while the report shows progress, she would like to see FDA pass on information to veterinarians and producers about the methodology of sales estimates, including a breakdown of use by livestock species. This could help establish a more functional baseline from which to measure future success.
Laurin says bovine sales numbers for penicillin and for aminoglycosides seem high in this report, but the report format leaves it unclear of which products are assigned to cattle use. She’s encouraged though, by the reductions in CTC sales.
Laurin also notes that year-to-year fluctuations in anaplasmosis incidence could significantly influence tetracycline use, as there are no other approved or effective treatments. Better data on use by species and purpose could help the industry correlate changes in usage patterns with natural fluctuations in disease incidence.
The VFD rules, Laurin says, have encouraged veterinarians, nutritionists and producers to focus more on nutrition, including minerals and vitamins, for preventing diseases such as foot rot. Feedstuffs used in rations often fall short in terms of vitamin quality and quantity, making supplementation critical for preventive management. At the same time though, recent shortages of vitamins A and E could be contributing to more antibiotic treatments.
Ohio veterinarian K. Fred Gingrich II, who serves as Executive Vice President of the American Association of Bovine Practitioners (AABP), says AABP’s guidelines for antimicrobial stewardship in bovine practice state that prevention to decrease the use of antibiotics and veterinary oversight of antimicrobial are important components of stewardship. AABP believes preventive practices can decrease overall antibiotic use in some situations, “however we exercise caution in having blanket targets for decreased antimicrobial use and prefer to focus on improving stewardship to decrease the unnecessary and injudicious use of antimicrobials on cattle farms and veterinary practices,” he says.
Gingrich also notes that AABP has long advocated for veterinary oversight of antimicrobial use. The AABP guideline for the veterinarian-client-patient relationship (VCPR) states that the veterinarian of record should have oversight of all antimicrobial use on the farm. The bovine veterinarian is the best resource for cattle farmers and ranchers to obtain guidance on the proper use of antimicrobials for their animals.
In the report, the FDA notes that sales and distribution information does not represent actual use of the products. Veterinarians and animal producers could, for example, purchase drugs but not actually administer them to animals or administer them in later years.
Critics often point to the quantity of antimicrobials used in food animals relative to use in human medicine, but FDA cautions that such comparisons can be misleading. There are for example, approximately 320 million people in the U.S., compared with about 9 billion chickens are slaughtered annually. Also, the average adult human weighs 182 pounds, compared with a finished beef steer weighing about 1,363 pounds. Finally, different animal species metabolize drugs differently, meaning that some may require more of the drug to be effective, or may need to be treated for a longer period of time.
It also is important to note that non-medically important antimicrobials account for virtually half of total antimicrobial sales for food animals. According to the report, sales of medically important antimicrobials for food animals during 2017 totaled 5,559,212 kilograms, compared with 5,374,156 kilograms for non-medically important products – 50.8% and 49.2% respectively.
“These reductions are an indication that our ongoing efforts to support antimicrobial stewardship are having a significant impact,” says FDA Commissioner Scott Gottlieb, M.D. “It’s important to note that this year’s report is the first to include sales/distribution data submitted after all medically important antimicrobial drugs administered to food-producing animals in their feed or water were no longer allowed to be used for growth promotion and could only be obtained through a veterinarian’s order.
Stewardship will evolve
While the 2017 report documents positive progress, the 2018 report likely will show further reductions in sales of antimicrobials for use in food animals. Implementation of GFI 213 and the VFD rules (since January 2017) have encouraged producers and veterinarians to shift toward more non-drug, management-oriented disease prevention.
The sales report, while helpful, serves only as an indicator, rather than direct evidence of progress. Actually it’s an indicator of an indicator. The goal of these efforts is to slow development of drug-resistant pathogens and protect the efficacy of antibiotics in human and veterinary medicine. Using antibiotic sales as a metric assumes:
- Antibiotic sales correlate with the volume of antibiotic use.
- The volume of antibiotic use in livestock, on its own, correlates with the incidence of resistance.
We know that all antibiotic use contributes, to some degree, to the development of resistant pathogens. We also know that “judicious use” entails more than simply reducing the volume of antibiotics administered. Diagnostics, dosage, duration of use along with complex interaction between the drug, animal and pathogen and other factors play key roles in resistance risk.
Ultimately, we need more information on actual resistance trends, ideally along with cause-and-effect data to direct best practices in judicious antibiotic use. The National Antibiotic Resistance Monitoring System (NARMS) is a collaborative program of state and local public health departments and universities, the FDA, the Centers for Disease Control and Prevention (CDC), and the USDA. That program tracks changes in the antimicrobial susceptibility of intestinal bacteria found in ill people (CDC), retail meats (FDA), and food animals (USDA). As the NARMS database grows, it becomes increasingly effective for identifying resistance trends.
The FDA sales data, along with trends reported from NARMS, certainly provide value in guiding industry decisions. Along with the reality of antibiotic resistance, public perception will drive future policy, whether regulatory or industry-driven. Consumers and activist groups clearly associate the volume of antibiotic use with the risk of resistance. In many cases, they also believe antibiotic residue in meat and dairy products is the culprit when resistance emerges among human pathogens. The new FDA report, showing substantial reductions in sales of medically important antibiotics for use in food animals over the past two years, helps demonstrate positive progress.







