Anaplasmosis Review: Part 1
By Gregg Hanzlicek, DVM, PhD, Director, Production Animal Field Disease Investigations, Kansas State Veterinary Diagnostic Laboratory
For this overview, I aim to address the most common questions about anaplasmosis we receive at the Kansas State diagnostic lab from our veterinary clients, and questions we hear from producers during field investigations. With anaplasmosis appearing to become more prevalent in some areas, cow-calf producers need awareness of clinical signs, vectors and preventive measures against death loss and abortions in their herds.
Anaplasmosis is caused by Anaplasma marinale in the United States. It is a gram negative bacteria and is considered to be an obligate intracellular organism. There are two forms; one form is intracellular and the other form survives outside the red blood cell. It makes sense that is has an extracellular form in order for it to infect other red blood cells or actually pass through the placenta to the fetuses.
All the strains we have identified, which probably is an incomplete list, are infective in cattle. Some of those strains, however, are not able to infect ticks. In areas where those strains are endemic, other modes of infection are more important than the ticks. Generally though, ticks are very important vectors for this disease.
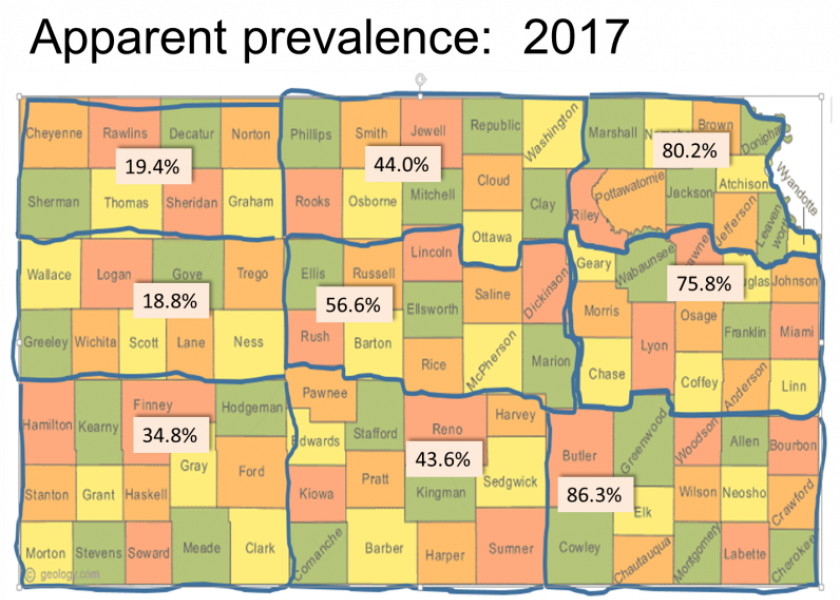
Seroprevalence
In an older but well-done study, University of Illinois researchers sampled almost all the counties in Illinois and found that 7% of all the cows they tested were positive for anaplasmosis. Other researchers collected samples from 1,800 randomly selected, 18-month and older animals at cattle markets in Texas. They found an overall prevalence of 15%, with a range of 5% to 15% in the east to as high as 20% to 38% in the west.
Through the Diagnostic Lab at K-State’s College of Veterinary Medicine, we recently completed a statewide anaplasmosis prevalence study, looking at nine agricultural districts in Kansas. We used USDA data to estimate the number of cows and herds in each district, and randomly selected veterinarians to participate in the study. We asked veterinarians who were eligible and willing to participate to provide lists of producers they believed would participate in the study without any idea if their herds were positive or negative. The Kansas State University College of Veterinary Medicine and Veterinary Medical
Research and Development, Pullman, Wash., (VMRD) helped fund the project.
We had excellent buy-in for the study, and ended up with 169 veterinarians participating. We aimed for a sample size of 900 herds, with 870 eventually participating. During the study, we ran ELISA analysis on herds that didn’t use the vaccine and used PCR testing for herds that did vaccinate against anaplasmosis.
Early results show the apparent seroprevalence at 70% to 80% in the eastern third of the state, which isn’t a big surprise for anyone who practices in those areas. Central Kansas had 43% to 56% prevalence, which is probably a little higher than what most people would expect. Results in the western part of the state shocked many veterinarians, producers and Extension specialists in the area. Prevalence in that area appears to range from 18% to 34%. The study included a survey for producers, and we are in the process of analyzing the survey results to determine what management practices are associated with seroprevalence and serostatus of the herds.
Strains and superinfection
There are at least 100 known genetic strains of A. marginale, and we know there are sub-strains beneath those. This is just a short list of known US strains from a couple of researchers.
Guy Palmer conducted an interesting study back in 2004, in which he tested an entire herd of 261 animals in Kansas. He found a prevalence of 29%, with 11 different strains in that one herd. Within that sample, 93% of those positive carried only one strain, while 7% carried two. Prior to that study, conventional wisdom suggested cattle could only carry one strain at a time.
Since that study, additional research demonstrating that cattle can carry more than one strain. Work in South America and Central America has shown herds in endemic areas are more likely to carry multiple strains than herd not in endemic areas. In our Kansas study, we captured blood from all study animals and currently are working to determine which strains are present.
Why do we care about strains? Because they differ in antigenic characteristics, transmission ability, infectivity, and pathogenicity. We know some strains cause very mild clinical signs and some strains have very severe clinical signs. Also, at least one study back in 2005 indicated that at least two strains have developed antimicrobial resistance.
Transmission
There are three modes of transmission for the Anaplasma pathogen: biological, mechanical, and transplacental.
For biological transmission, the primary vectors are ticks, which serve as amplifiers. When ticks feed on a positive animal, the bacteria establish inside the tick reproduce, primarily in the hind gut and the salivary glands, and the concentration can reach very high levels. When the tick feeds on an animal it will pass those bacteria through the saliva. There are four species of ticks in the US that carry this disease, all in the Dermacenter genus. Not all ticks in the US carry Anaplasma. In Kansas for example, we have many Lone Star ticks, which carry other pathogens but not Anaplasma.
For most of the United States, the American Dog Tick is the most common species to transmit Anaplasma to cattle. The male ticks are the primary vectors, because they are intermittent feeders. The female attaches, fills up with blood, gets inseminated and falls off before laying eggs. The males on the other hand, feed on a host and if they don’t find a female, they can drop off and latch onto another animal. One of our tick researchers, who spends time in the field looking for ticks, collected 200 ticks from 12 different locations in the eastern half of Kansas back in 2004. In his sampling, 33% of those ticks tested positive for Anaplasma through PCR analysis. He has repeated it several times. So within the tick population in the eastern part of Kansas, we can assume a high percentage of American Dog Ticks are positive for anaplasmosis.
The second mode of transmission is mechanical, mostly involving horse, stable, and deer flies. Horn flies are not thought to be a major mover of this disease amongst populations. Flies don’t amplify the organism like the ticks do, so the bacteria those flies pick up when feeding is the maximum they will be able to pass on to the next animal when they take their blood meal. They do pass it, but they don’t amplify it. Research has shown ticks are more efficient at transmitting this disease than flies, which makes sense if they are replicating in the tick and the concentration is higher.
We receive numerous calls from producers asking about flies, how far they travel and how long can they carry the pathogen. In one study, researchers allowed flies to feed on a positive animal, and then they let them feed on splenectomized calves. In that study, the organisms remained alive in flies anywhere from three minute to two hours, allowing plenty of time for a fly to carry the bacteria from one animal to another.
Distance traveled is a major issue for managing this disease, especially in endemic areas. In a study all the way back in 1940, researchers tagged and released horse flies and were able to find them three to eight miles away. A more recent study in 1976 found 80% of captured horse flies within a half-mile of the release point. They didn’t say where they found the other 20% or if they even tried. The point is these flies can travel a fair distance and certainly across fences. Again, for those of you trying to manage Anaplasma in herds this is always a consideration.
Fomites also play a role in mechanical transmission. We do know that needles are very effective in transmitting this disease. Some studies indicate a palpation sleeve and ultrasound probes can pass viral pathogens, but I haven’t seen any studies that show these pass Anaplasma. It seems plausible though, because of the possibility of moving positive blood from one animal to another.
Syringes have been shown to spread Anaplasma. A study by Reinbold in 2010 used a positive Anaplasma animal, with just 2% of its red blood cells infected, meaning a relatively low level of infection. The researchers injected 10 negative animals, after first using the needle on the positive animal, and without cleaning, disinfecting or changing needles. In between injecting each negative animal they injected the positive one. Only on the last injection did they even notice there was any blood on the needle.
They came back after an appropriate time and six out of those 10 became positive just from the movement of the needle from a positive with a very low bacterimic level to negative animals. It doesn’t take much to pass this organism to naïve animals. At least from this study, if you have a positive animal in the chute, and you have a negative animal behind it and you’re not disinfecting or changing needles, there is a 60% probability that the animal behind it will become positive. Needles are a big deal in positive herds.
Part 2 of this series will explore transpacental transfer, temporal effects, types of infection, clinical signs, diagnostic practices treatments and prevention of anaplasmosis.







